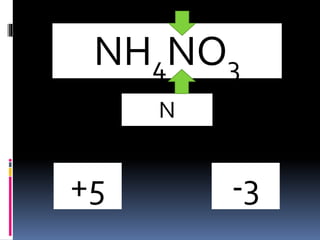
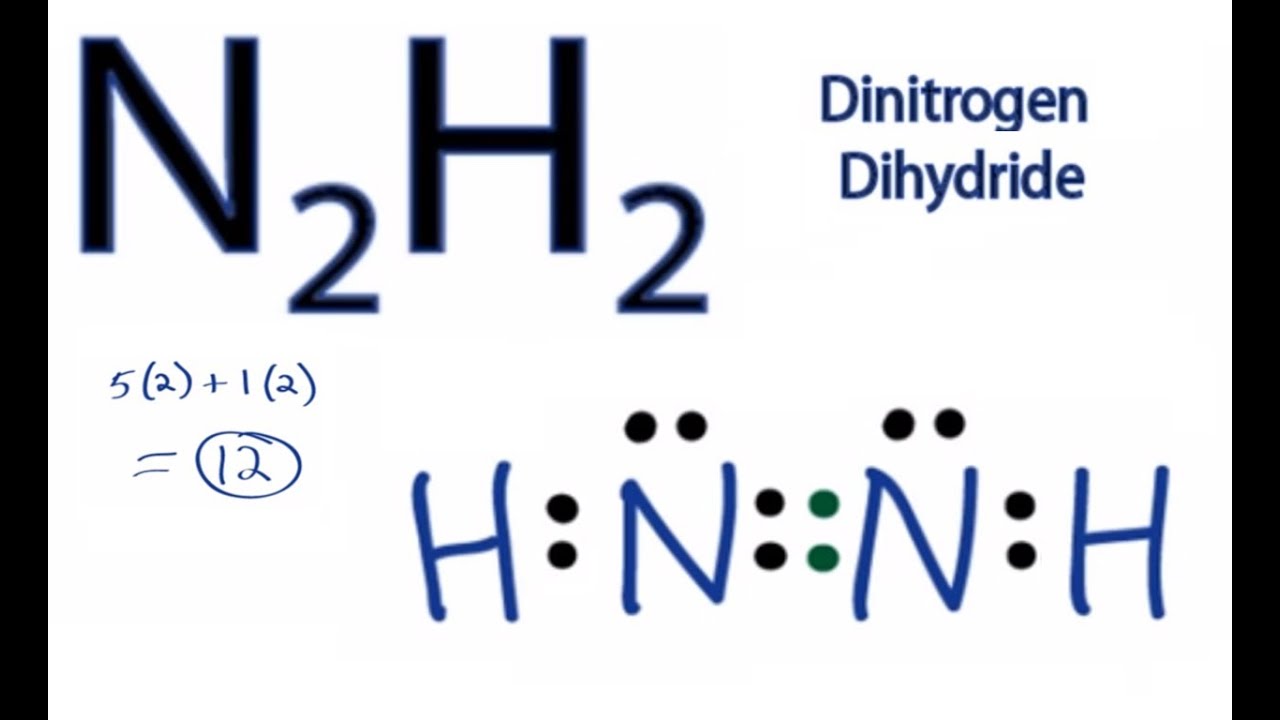60 POINTS - If the item is not a molecule, indicate that it is not a molecule. - If it is a molecule, - brainly.com
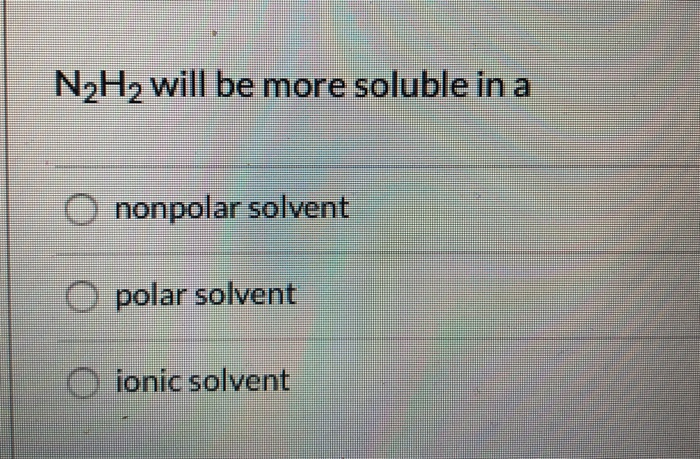
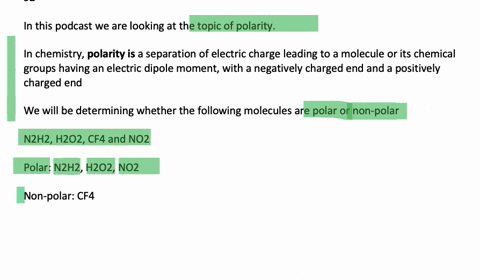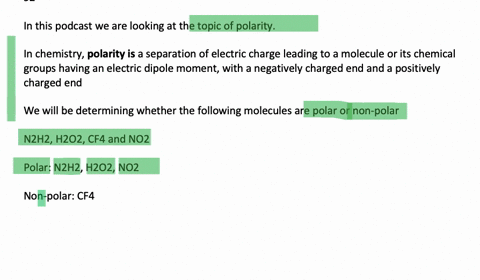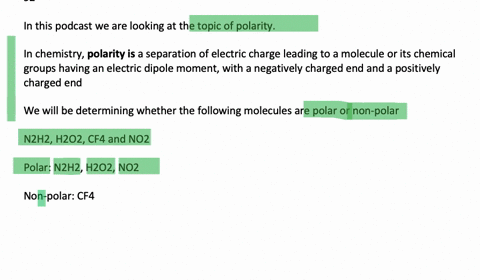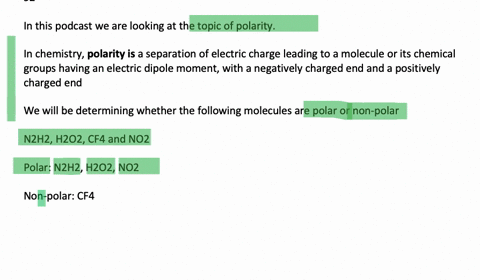
N2H2 is polar molecule with london dispersion forces,dipole dipole forces and H bonding .True or false?How?

N2H2 Molecular Geometry, Bond Angles & Electron Geometry (Diimide) | N2H2 Molecular Geometry, Bond Angles & Electron Geometry (Diimide) Molecules like N2H2 can be a little tricky because although they might seem

Plasma-Catalyst Reactivity Control of Surface Nitrogen Species through Plasma-Temperature-Programmed Hydrogenation to Ammonia | ACS Sustainable Chemistry & Engineering